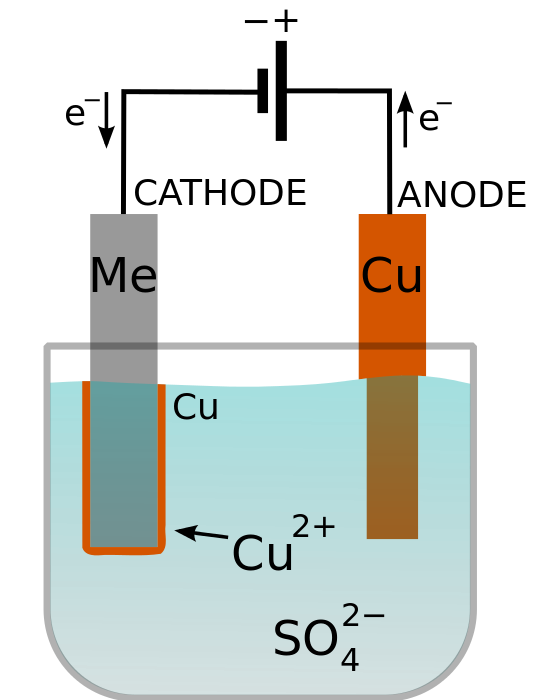
Copper Sulfate Conductivity . to observe electrical conductivity of substances in various aqueous solutions; the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. the specific conductivities and ph values of copper sulphate solutions in water were measured in the temperature range from. aqueous solutions of copper (ii) sulfate have been studied by dielectric relaxation spectroscopy (drs) over a wide range of frequencies (0.2 less, similar nu/ghz < or =. much of the confusion in copper sulphate solution resistivity measurement derives, it seems to me, from surface insulating films. To determine of the solution is a strong or weak electrolyte; In this experiment1, you will determine whether or not several solutions are electrolyte solutions,.
from pediaa.com
to observe electrical conductivity of substances in various aqueous solutions; To determine of the solution is a strong or weak electrolyte; In this experiment1, you will determine whether or not several solutions are electrolyte solutions,. the specific conductivities and ph values of copper sulphate solutions in water were measured in the temperature range from. much of the confusion in copper sulphate solution resistivity measurement derives, it seems to me, from surface insulating films. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. aqueous solutions of copper (ii) sulfate have been studied by dielectric relaxation spectroscopy (drs) over a wide range of frequencies (0.2 less, similar nu/ghz < or =.
Difference Between Electrolysis and Electroplating Definition
Copper Sulfate Conductivity to observe electrical conductivity of substances in various aqueous solutions; aqueous solutions of copper (ii) sulfate have been studied by dielectric relaxation spectroscopy (drs) over a wide range of frequencies (0.2 less, similar nu/ghz < or =. To determine of the solution is a strong or weak electrolyte; In this experiment1, you will determine whether or not several solutions are electrolyte solutions,. the specific conductivities and ph values of copper sulphate solutions in water were measured in the temperature range from. to observe electrical conductivity of substances in various aqueous solutions; the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. much of the confusion in copper sulphate solution resistivity measurement derives, it seems to me, from surface insulating films.
From fphoto.photoshelter.com
science chemical reaction equilibrium cupric sulfate pentahydrate Copper Sulfate Conductivity the specific conductivities and ph values of copper sulphate solutions in water were measured in the temperature range from. much of the confusion in copper sulphate solution resistivity measurement derives, it seems to me, from surface insulating films. aqueous solutions of copper (ii) sulfate have been studied by dielectric relaxation spectroscopy (drs) over a wide range of. Copper Sulfate Conductivity.
From www.youtube.com
Timelapse of copper sulfate electrolysis YouTube Copper Sulfate Conductivity To determine of the solution is a strong or weak electrolyte; In this experiment1, you will determine whether or not several solutions are electrolyte solutions,. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. to observe electrical conductivity of substances in various aqueous solutions; aqueous solutions. Copper Sulfate Conductivity.
From www.researchgate.net
Fig. S10 UVVis spectrum of copper(II) sulfate. Download Scientific Copper Sulfate Conductivity In this experiment1, you will determine whether or not several solutions are electrolyte solutions,. to observe electrical conductivity of substances in various aqueous solutions; the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. To determine of the solution is a strong or weak electrolyte; much of. Copper Sulfate Conductivity.
From www.youtube.com
Copper II Sulfate Electroconductivity Probe YouTube Copper Sulfate Conductivity aqueous solutions of copper (ii) sulfate have been studied by dielectric relaxation spectroscopy (drs) over a wide range of frequencies (0.2 less, similar nu/ghz < or =. To determine of the solution is a strong or weak electrolyte; to observe electrical conductivity of substances in various aqueous solutions; much of the confusion in copper sulphate solution resistivity. Copper Sulfate Conductivity.
From www.researchgate.net
Graphs of the thermal conductivity in the diamondcopper system Copper Sulfate Conductivity To determine of the solution is a strong or weak electrolyte; the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. much of the confusion in copper sulphate solution resistivity measurement derives, it seems to me, from surface insulating films. aqueous solutions of copper (ii) sulfate have. Copper Sulfate Conductivity.
From www.researchgate.net
Effect of copper sulphate concentration on wt of copper in alloy. SEM Copper Sulfate Conductivity In this experiment1, you will determine whether or not several solutions are electrolyte solutions,. much of the confusion in copper sulphate solution resistivity measurement derives, it seems to me, from surface insulating films. to observe electrical conductivity of substances in various aqueous solutions; To determine of the solution is a strong or weak electrolyte; aqueous solutions of. Copper Sulfate Conductivity.
From blog.thepipingmart.com
Thermal Conductivity Of Copper Copper Sulfate Conductivity to observe electrical conductivity of substances in various aqueous solutions; the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. To determine of the solution is a strong or weak electrolyte; In this experiment1, you will determine whether or not several solutions are electrolyte solutions,. much of. Copper Sulfate Conductivity.
From www.echemi.com
Molar Absorptivity of Copper(II) Sulfate ECHEMI Copper Sulfate Conductivity to observe electrical conductivity of substances in various aqueous solutions; much of the confusion in copper sulphate solution resistivity measurement derives, it seems to me, from surface insulating films. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. aqueous solutions of copper (ii) sulfate have. Copper Sulfate Conductivity.
From www.alamy.com
Electrolysis of copper sulphate solution using copper electrodes Stock Copper Sulfate Conductivity aqueous solutions of copper (ii) sulfate have been studied by dielectric relaxation spectroscopy (drs) over a wide range of frequencies (0.2 less, similar nu/ghz < or =. to observe electrical conductivity of substances in various aqueous solutions; the specific conductivities and ph values of copper sulphate solutions in water were measured in the temperature range from. . Copper Sulfate Conductivity.
From www.vecteezy.com
Electrolysis of copper sulfate solution with impure copper anode and Copper Sulfate Conductivity the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. To determine of the solution is a strong or weak electrolyte; much of the confusion in copper sulphate solution resistivity measurement derives, it seems to me, from surface insulating films. In this experiment1, you will determine whether or. Copper Sulfate Conductivity.
From mungfali.com
Electrical Conductivity Diagram Copper Sulfate Conductivity the specific conductivities and ph values of copper sulphate solutions in water were measured in the temperature range from. aqueous solutions of copper (ii) sulfate have been studied by dielectric relaxation spectroscopy (drs) over a wide range of frequencies (0.2 less, similar nu/ghz < or =. to observe electrical conductivity of substances in various aqueous solutions; . Copper Sulfate Conductivity.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte Copper Sulfate Conductivity aqueous solutions of copper (ii) sulfate have been studied by dielectric relaxation spectroscopy (drs) over a wide range of frequencies (0.2 less, similar nu/ghz < or =. to observe electrical conductivity of substances in various aqueous solutions; much of the confusion in copper sulphate solution resistivity measurement derives, it seems to me, from surface insulating films. To. Copper Sulfate Conductivity.
From www.youtube.com
Explain the nature of thermogram of Copper Sulphate Pentahydrate (CuSO4 Copper Sulfate Conductivity to observe electrical conductivity of substances in various aqueous solutions; To determine of the solution is a strong or weak electrolyte; aqueous solutions of copper (ii) sulfate have been studied by dielectric relaxation spectroscopy (drs) over a wide range of frequencies (0.2 less, similar nu/ghz < or =. the following table gives the electrical conductivity of aqueous. Copper Sulfate Conductivity.
From stock.adobe.com
Molecular formula of copper sulfate. Chemical structure of copper Copper Sulfate Conductivity aqueous solutions of copper (ii) sulfate have been studied by dielectric relaxation spectroscopy (drs) over a wide range of frequencies (0.2 less, similar nu/ghz < or =. In this experiment1, you will determine whether or not several solutions are electrolyte solutions,. the specific conductivities and ph values of copper sulphate solutions in water were measured in the temperature. Copper Sulfate Conductivity.
From www.odinity.com
Determination of Cupric Sulfate Hydration Number Odinity Copper Sulfate Conductivity In this experiment1, you will determine whether or not several solutions are electrolyte solutions,. to observe electrical conductivity of substances in various aqueous solutions; the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. aqueous solutions of copper (ii) sulfate have been studied by dielectric relaxation spectroscopy. Copper Sulfate Conductivity.
From www.youtube.com
Color of Copper (II) sulfate [hydrous and anhydrous] YouTube Copper Sulfate Conductivity the specific conductivities and ph values of copper sulphate solutions in water were measured in the temperature range from. much of the confusion in copper sulphate solution resistivity measurement derives, it seems to me, from surface insulating films. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function. Copper Sulfate Conductivity.
From sanctamariascience.blogspot.com
Science Sancta Maria College 1st Year Science Copper Sulfate Conductivity the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. the specific conductivities and ph values of copper sulphate solutions in water were measured in the temperature range from. aqueous solutions of copper (ii) sulfate have been studied by dielectric relaxation spectroscopy (drs) over a wide range. Copper Sulfate Conductivity.
From www.doubtnut.com
Copper sulphate solution is electrolysed using copper electrodes. Copper Sulfate Conductivity In this experiment1, you will determine whether or not several solutions are electrolyte solutions,. much of the confusion in copper sulphate solution resistivity measurement derives, it seems to me, from surface insulating films. the following table gives the electrical conductivity of aqueous solutions of some acids, bases, and salts as a function of. the specific conductivities and. Copper Sulfate Conductivity.